Procurement Outsourcing in the Pharma Industry: Risks and Rewards
Pharmaceutical Procurement Services: Navigating a New Operational Landscape
Pharmaceutical procurement services are no longer only practiced behind the scenes at pharmaceutical headquarters. In an industry requiring significant market responsiveness, quality assurance and regulatory adherence, procurement has evolved into an important front-line function. Over the last few years, the compounding complexity of pharmaceutical supply chains has triggered a rethink of procurement models with many companies now looking at external procurement service providers.
Previously, the pharmaceutical companies were in control of basic procurement practices by managing daily business relationships with vendors, conducting audits and compliance documentation along with quality assurance functions. Although this led to enhanced control, it also created longer decision cycles and greater operational overheads. When procurement functions are outsourced to specialized service partners it opens pathways for global supplier opportunities, shorter procurement cycles and advancements in the depth of category expertise. The new strategic imperatives in procurement are particularly appealing to mid-sized companies and generic pharmaceutical manufacturers who historically do not possess a depth of procurement infrastructure.
However, shifting from a traditional procurement model is more than simply an exercise in cost control. As a highly regulated industry, procurement affects all aspects of the pharmaceutical product-development lifecycle, particularly drug safety, traceability and patent safety. As a result, pharmaceutical procurement services must be sourced and managed with surgical precision because any neglect could risk compromising a product or a patient’s safety.
Pharma Supply Chain Outsourcing: Strategic Advantage or Structural Gamble?
As drug companies expand into new geographies, the benefits of outsourcing the logistics function of their pharma supply chain become even more attractive. If drugs are produced somewhere else, then the outsourcing of their logistics, vendor qualification, procurement, and even quality audits to a third-party vendor offers firms the opportunity to scale up quickly and more flexibly. In low-cost sourcing geographies like India, China, and portions of Southeast Asia, drug manufacturing presents compelling benefits actively managing cost and moving fast.
That said, the risks are also very real. In an environment where a failure in quality or compliance can lead to the stoppage of a drug product line, additional aggravation must be added to the stringent oversight of pharma supply chain outsourcing. For example, the most recent API shortages in Europe (2022) were partly due to reliance on external procurement partners that lacked visibility over secondary- and tertiary-suppliers. The pharma companies that used these vendors or had vendor ecosystems with limited vendor direct control, were left looking for alternatives.
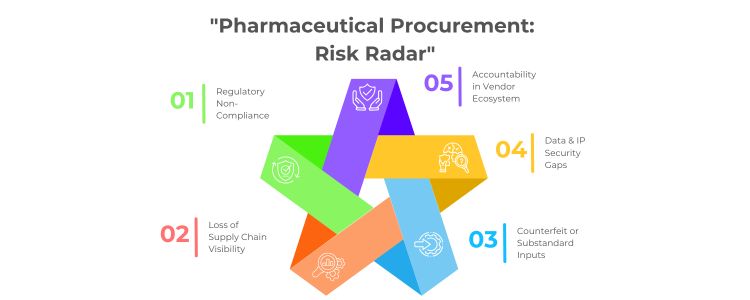
Of additional concern, outsourcing is outwardly amorphous in terms of accountability. If a batch of product fails post market inspection due to defective ingredients, who is responsible? Accountability is often articulated in the terms of a contract or a binary email chain. This lack of accountability can lead to delays in investigations, brand damage, and financial impacts.
Pharmaceutical Sourcing Trends 2025: A Shift Toward Intelligence and Sustainability
Looking ahead, the trends in pharmaceutical sourcing slated for 2025 demonstrates not only how pharmaceutical companies will develop their sourcing and procurement strategies, but also how sourcing is moving from a transactional reactive buying process to become an intelligence-led ecosystem approach to risk mitigation with outsourcing in mind; driven by three primary factors within the industry: digital transformation, sustainability expectations, and geopolitical realignments relevant to the industry.
One impact of digital transformation is sourcing and procurement moving toward a complete digital alternative; e-procurement solutions that operate on a cloud-based premise, AI-enhanced supplier accreditation and evaluation; and blockchain enabled traceability solutions are all examples of alternatives to how pharmaceutical firms can manage outsourced procurement, providing transparency and immediate pre-existing compliance anomalies that help to mitigate supply chain risk.
Sourcing trends 2025 also delineate a shift to sustainable supplier selection criteria, where increasing numbers of companies are opting for suppliers that align to Key Performance Indicators connected to their ESG commitments and sourcing local raw materials to alleviate carbon footprint impacts. Supply conversion within sourcing categories with high order volumes, including surgical equipment, items and medical disposables to cite a few industries, have also seen a resurgence of local vendor development in regions of Latin American, Eastern European and South Asian geographies.
Trends in Apr 2023 show a growing interest towards hybrid operational models. Hybrid operational models allow for strategic sourcing of in-house processes (i.e. APIs and rare compounds); whilst outsourcing processes required for operational procurement; thus allowing firms to retain ownership of critical product inputs while accelerating new operational efficiencies to scale as an outsourced part of transportation of drugs.
In sum, the future of procurement in pharma is not about outsourcing or insourcing. It’s about creating a fit-for-purpose procurement ecosystem that can adapt to change while upholding safety, compliance, and commercial goals.
Managing the Risks: What Can Go Wrong?
Outsourcing the procurement function in pharma has significant potential benefits but it approaches a high-stakes game.
Here are some of the most significant risks that companies must plan for and mitigate:
- Regulatory non-compliance: Third party vendors may not have a thorough understanding of regulatory requirements in the target markets which can result in batch disqualifications, import holds or permanent bans from markets.
- Loss of visibility: The third-party provider may use their own technology platforms to facilitate buying, outsourcing limits oversight at the category and supplier level with respect to the allocation of cost in the procurement process, supplier performance, or ethics in procuring supplier materials.
- Counterfeit or inferior products exposure: The complexity of outsourced models with multiple subcontractors and suppliers, increases the opportunity for counterfeiting or inferior raw materials entering the value chain into manufacturing.
- Data security: If you are dealing with an R&D pipeline or formulation data, third-party service providers lacking strong cybersecurity protocols may increase risk of intellectual property theft.
A well-defined pharma procurement program must establish comprehensive onboarding procedures for its vendors, conduct periodic compliance audits, and leverage technology to afford full traceability and provenance of resources consumed from its suppliers. Service level agreements (SLA) should be framed to reward compliance with regulatory standards and quality outputs, not just cost savings. Moreover, internal teams must be trained to manage the outsourced function, not just execute the procurement function where procurement value is defined narrowly as a transaction or purchase for a product or service. This is where the procurement function is shifting from a transactional function to value add as a strategic business function.
The Rewards: Efficiency, Scale, and Strategic Insight
Procurement outsourcing, when properly managed, should provide visible gains in the efficiency and scalability of your procurement operations. Similar to direct sourcing, shorter procurement cycle times, better responsiveness to suppliers and greater leverage on pricing are several immediate benefits.
However, procurement outsourcers generally come with years of category experience and supplier knowledge that internal teams would have to build over the years to develop. Situations requiring sourcing a complex API or niche categories requiring exacting quality certifications or geo-specific ingredients, would be examples where external procurement resources with experience and contacts could be beneficial.
Pharma companies are also relieved of using internal resources for administrative, tactical and regional procurement. Providing the freedom to focus on R&D and core manufacturing, an outsourced procurement structure allows you to respond faster and do more things relative to your go-to-market timeline in today’s environment where timing to market can mean the difference between competitive advantage.
Even for large companies structured properly, taking advantage of external service providers providing benchmarking from within their category expertise and intelligence can help free up hidden value, improve tail-end spend and better negotiated long term contracts.
Governance and Future Outlook
Effective governance is an essential first step in securing sustainability in outsourcing. It is important to set the groundwork for the relationship in terms of transparency, accountability, and compliance in the beginning. Some examples are: these consensus agreements can be emphasized by defining contract language regarding risk-sharing, defining transparent reporting and accountability, and agreeing to escalation protocols.
In order to be sustainable, procurement must be in a constant state of improvement. There are constant changes to regulatory frameworks, market equilibrium, and technology improvements hence procurement models must be catalyzed against constantly. Procurement sustainability is dependent on the agreement being adaptable and flexible. A static agreement in a dynamic industry will see the agreement stagnate, and then so too will the organization.
In the pharmaceutical sector, the heavy regulation and mission-driven mandate to deliver safe products can be daunting. In our industry, one lapse in procurement cannot be undone the same way it can in other industries. A single procurement lapse leading to a resolution can have unforeseen consequences related to security, legal reprimand, and finally the demise of the total supply chain for patients.
Conclusion
Procurement outsourcing in the pharma sector is a complicated decision. It is neither a guaranteed solution nor an automatic risk. It is a complex decision that will create operational value for the enterprise, enhance operational agility, and allow for a deeper set of global capabilities when executed correctly.
As pharmaceutical sourcing trends 2025 challenge what is meant to be efficient, digitally integrated, and focused on ESG, the best companies and professionals will consider procurement as not simply a cost center, but as a value creating and sustaining competitive weapon for their business.
Pharmaceutical procurement services and reasonably constructed pharma supply chain outsourcing will remain fundamental to an uncertain global market consisting of reasonable demand for safe, effective and sustainable products.









